-

-
OTTAWA, ON, December 23, 2020 – OraSure Technologies, Inc. (NASDAQ: OSUR), a leader in point-of-care diagnostic tests, specimen collection devices, and microbiome laboratory and analytical services, today announced that its OMNIgene®·ORAL (OM/OME-505) collection device was included in the U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA) amendment granted to 3B BlackBio Biotech, one of the leading suppliers and developers for RT-PCR diagnostic assays in India. This is the seventh customer EUA that includes a collection device from the company’s DNA Genotek subsidiary.
-

-
BETHLEHEM, PA, November 3, 2020 - OraSure Technologies, Inc. (NASDAQ: OSUR), a leader in point-of-care diagnostic tests, specimen collection devices, and microbiome laboratory and analytical services, today announced its DNA Genotek subsidiary has received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for the use of the ORAcollect®·RNA (OR/ORE-100) saliva collection device for the collection, stabilization and transport of saliva specimens suspected of containing SARS-CoV-2 RNA. This is the second FDA EUA that DNA Genotek has received for its saliva collection devices.
-

-
BETHLEHEM, Pa., September 24, 2020 - OraSure Technologies, Inc. (NASDAQ: OSUR), a leader in point-of-care diagnostic tests, specimen collection devices, and microbiome laboratory and analytical services, today announced that its ORAcollect®·RNA (OR-100) saliva collection device has been included in the U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA) granted to Quadrant Biosciences Inc. for a COVID-19 laboratory test.
-

-
Pure Culture Beauty has partnered with DNA Genotek and Diversigen, the leading providers of high-quality biological sample collection products and end-to-end services for genomics and microbiome applications. DNA Genotek will supply the OMNIgene·SKIN microbiome collection kits and Diversigen will provide full microbiome analysis services, offering a complete understanding of the users’ skin microbiome. DNA Genotek and Diversigen are wholly owned subsidiaries of OraSure Technologies, Inc. (NASDAQ: OSUR).
-

-
OraSure’s ORAcollect®·RNA Device Included in EUA Granted to MiraDx Inc. for SARS-CoV-2 Test
BETHLEHEM, PA -- September 3, 2020 - OraSure Technologies, Inc. (NASDAQ: OSUR), a leader in point-of-care diagnostic tests, specimen collection devices, and microbiome laboratory and analytical services, today announced that its ORAcollect®·RNA (OR-100) collection device was included along with other devices in the U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA) granted to MiraDx Inc., a Los Angeles-based molecular genetics company. This is the fifth EUA to include a collection device from the company’s DNA Genotek subsidiary.
-

-
BETHLEHEM, PA, July 31, 2020 - OraSure Technologies, Inc. (NASDAQ: OSUR), a leader in point-of-care diagnostic tests, specimen collection devices, and microbiome laboratory and analytical services, today announced that its OMNIgene®·ORAL (OM-505) saliva collection device is included in the U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA) granted to Clinical Reference Laboratory (CRL), one of the largest privately held clinical testing laboratories in the United States, for SARS-CoV-2 testing. This is the fourth EUA to include a collection device manufactured and sold by the Company’s DNA Genotek subsidiary.
-

-
DNA Genotek unveils first in-home self-collection device for Metabolomics
OTTAWA, ON, June 17, 2020 - OraSure Technologies, Inc. (NASDAQ: OSUR), a leader in point-of-care diagnostic tests, specimen collection devices, and microbiome laboratory and analytical services is introducing the first and only commercially available device for in-home, self-collection of fecal samples for metabolomics. A product of OraSure’s DNA Genotek subsidiary, the OMNImet™·GUT (ME-200) augments the Company’s portfolio of multiomic sample collection products. The device is intended for research use only.
-

-
Oragene®·Dx included in EUA allowing at-home saliva collection for Phosphorus SARS-CoV-2 test
OTTAWA, ON, June 8, 2020 - OraSure Technologies, Inc. (NASDAQ: OSUR), a leader in point-of-care diagnostic tests, specimen collection devices, and microbiome laboratory and analytical services, announced today that Phosphorus Diagnostics, a leader in diagnostic and bioinformatic solutions for clinical next generation sequencing (NGS)
-

-
OTTAWA, ON, May 22, 2020 - OraSure Technologies, Inc. (NASDAQ: OSUR), a leader in point-of-care diagnostic tests, specimen collection devices, and microbiome laboratory and analytical services, (NASDAQ: OSUR), announced today that its OMNIgene®·ORAL saliva collection device (OM-505) was included in the U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA) recently granted to P23 Labs. This EUA permits individuals to self-collect saliva specimens at-home for the detection of SARS-CoV-2.
-
-
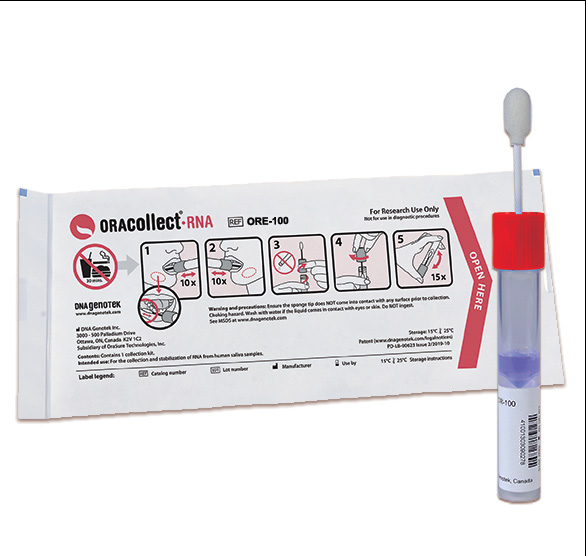
DNA Genotek's ORAcollect®·RNA kit included in EUA granted to Biocerna SARS-CoV-2 test
OTTAWA, ON, May 4, 2020 - DNA Genotek Inc., a leading provider of sample collection kits and end-to-end services and a wholly-owned subsidiary of OraSure Technologies, Inc. (NASDAQ: OSUR), announced today that its ORAcollect®·RNA kit (OR-100) was included as the collection device for anterior nares (nasal) samples under a U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA)granted to Biocerna LLC for its PCR-based SARS-CoV-2 assay.
Press Releases
2020
Dec 23
Nov 03
Sep 24
Sep 15
Sep 03
July 31
June 17
June 08
May 22
May 04
January 22
DNA Genotek Announces FDA General Use Clearance for Oragene®·Dx Family of Products
OTTAWA, ON, January 22, 2020 - DNA Genotek Inc., a leading provider of sample collection kits and end-to-end services and a wholly-owned subsidiary of OraSure Technologies, Inc. (NASDAQ: OSUR), announced today that the U.S. Food and Drug Administration (FDA) has granted a general use 510(k) clearance for its Oragene®•Dx family of products.

