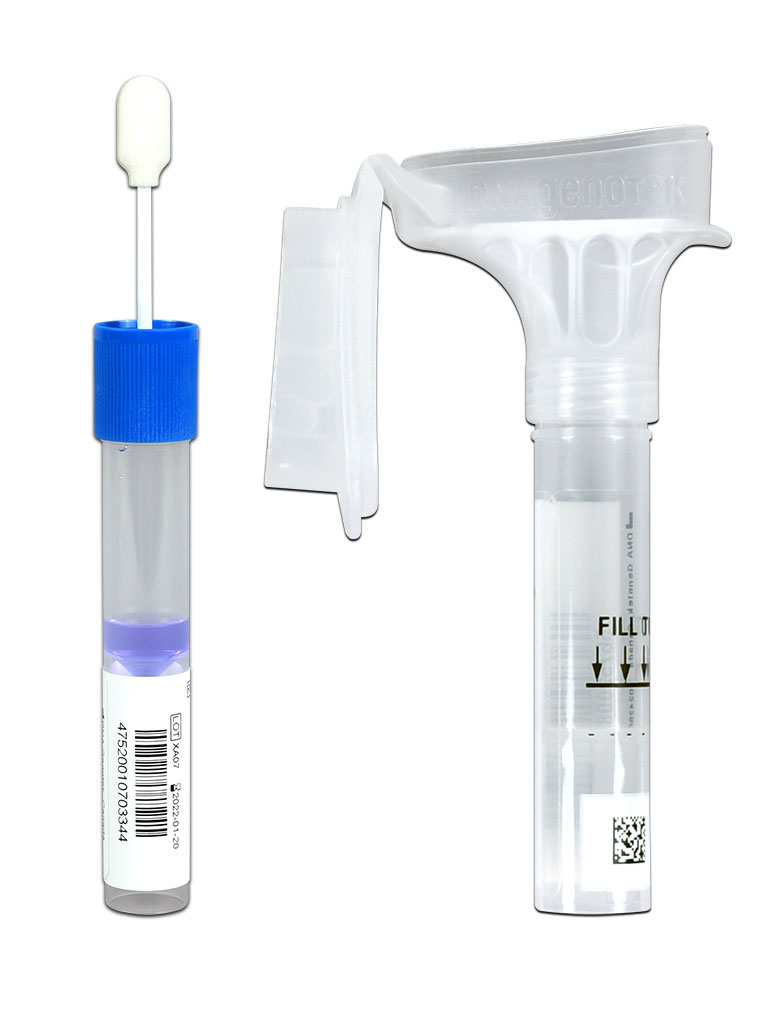
EVALUATE U.S. FDA 510(k)-CLEARED SALIVA COLLECTION DEVICES FOR YOUR GENETIC TEST SYSTEM
As a strategic collaborator to our customers, we offer resources and expertise to clinical labs on navigating any changes to the regulatory pathway effectively.

Sample collection devices play a crucial role in ensuring reliable and high-quality samples for diagnostic testing applications. The use of U.S. FDA-cleared devices provides assurances regarding safety, effectiveness and quality of that component, which helps assure safety and effectiveness of the test system
Advantages of using FDA-cleared devices:
Consider integrating FDA-cleared Oragene™•Dx and ORAcollect™•Dx saliva collection devices into your genetic test system. These devices have 510(k) general clearance for prescription and over-the-counter use for the collection, stabilization and transportation of DNA from saliva at ambient temperatures
Expert support from DNA Genotek Inc.:
Leveraging our FDA-cleared devices and regulatory expertise can help labs meet regulatory requirements more efficiently, reducing the need for extensive in-house regulatory resources
To request free samples of Oragene●Dx or ORAcollect●Dx devices or to request a quote/consultation, please contact DNA Genotek Inc. here
| REQUEST QUOTE/SAMPLES OF Oragene●Dx AND ORAcollect●Dx DEVICES |
|
*The Oragene™•Dx device and ORAcollect™•Dx device direct-to-consumer (DTC) user comprehension studies (K192920 and K212745, respectively) were conducted to support a pre-market submission to the FDA. These studies were conducted to assess the acceptability of saliva samples collected with the Oragene™•Dx or ORAcollect™•Dx devices for a direct-to-consumer application. The objective of the studies was to demonstrate that naive participants comprehend the instructions for use provided with the devices and can successfully provide a saliva sample at home with acceptable quality for use in downstream diagnostic testing applications.
Some DNA Genotek products may not be available in all geographic regions. Customers must review the labels (RUO or IVD) of sample collection devices and ensure compliance with their intended use. Device / Assay manufacturers must validate the use of Oragene™•Dx and ORAcollect™•Dx for their specific indications for use
Check product availability in your region by selecting your location on the website. Full terms and conditions for all DNA Genotek products are available here. DNA Genotek's sample collection devices and nucleic acid stabilization chemistries are protected by issued and pending patents in numerous countries around the world.
Inside DNA Genotek
Legal